EuRx Pharma | –40% Recall Costs & ROI in Less Than 9 Months Thanks to Tamper-Proof Laser Marking
Executive Summary
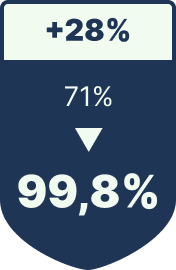
EU anti-counterfeiting regulations require full serialization—but paper labels often fail under real-world cold-chain conditions. EuRx Pharma replaced adhesive labels with direct laser marking on blister foils, vials, and ampoules. The permanent code withstands abrasion, IPA-based disinfectants, and –80 °C storage, while achieving a 99.8% first-pass read rate during inline inspection.
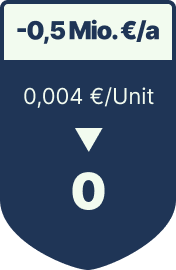
The result: 40% fewer recall-related costs, €0.5M in annual OPEX savings, and full payback in under nine months.

Context / Persona
- Industry: EU-based R&D-driven Rx pharmaceutical manufacturer (solid & liquid forms)
- Role: Head of Packaging Operations
- Objective: Zero label-related deviations across 120 million units per year
Challenge
Falsified or mislabelled medications cost global healthcare systems an estimated $30.5 billion annually. Labels detach in over 70% of temperature-sensitive products during cold-chain transit.
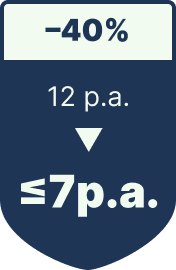
Each unit without a scannable code risks regulatory fines, blocked deliveries, and costly recalls—1,284 drug recalls per year on average, 14.9% of which are caused by labeling errors.
Solution Approach
A UV laser engraves a GS1-compliant DataMatrix code directly into the blister lid foil, glass body, or aluminum ampoule. The high-contrast micro-engraving is permanent and resistant to chemicals.
A camera verifies every code. Data is transferred to the Level 4/5 serialization database, building a digital twin of each batch.
Eliminating ink, labels, and solvents reduces costs and VOC emissions.
Any manipulation leaves visible microfractures in the engraved code—offering built-in tamper detection.
Implementation Roadmap
| Phase | Duration | Key Stakeholders | Effort* |
|---|---|---|---|
| ROI Analysis & Business Case | 4 weeks | Packaging, QA, Finance | 2 FTEs |
| Technical Pilot (1 blister + 1 vial line) | 8 weeks | Engineering, IT | 3 FTEs |
| Scaling to 5 lines incl. aggregation | 12 weeks | OT/IT team, vendors | 4 FTEs |
| GMP Validation & Regulatory Approval | 4 weeks | QA, RA | 2 FTEs |
Key Metrics
First-Pass-Read-Rate
Label-Related Recalls
Consumable OPEX
(Key Performance Indicator, delta, baseline, result)