Tamper-Proof Laser Marking – Permanent Traceability for Metallic Implants and Surgical Instruments
Executive Summary
Senodis provides tamper-proof, sterilization-resistant laser marking specifically developed for metallic implants and surgical instruments. These markings comply with MDR and UDI regulations, enable full digital traceability—and reduce recall and process costs so effectively that ROI is typically achieved within six months.
With Senodis Laser ID, you turn traceability from a regulatory burden into a competitive advantage—secure, audit-ready, and measurably profitable.

Challenge
- Stricter MDR Requirements
Since 2021 (Class III) and starting 2025 (Class I), the EU Medical Device Regulation (MDR) mandates permanent direct UDI marking on all critical medical devices. Traditional adhesive labels or ink-based markings cannot withstand repeated sterilization cycles. - Recall Risk & Legal Exposure
On average, medical implant recalls cost up to $600 million, with poor traceability often being a root cause of liability and cost escalation. - Counterfeit Components
Fake spare parts and third-party implants pose serious safety risks and undermine brand trust. The EUIPO estimates annual losses of over €1.4 billion within the European single market alone.
Solution
| Feature | Benefit for QA/RA & Supply Chain |
|---|---|
| Sterilization-Resistant (≥ 500 Cycles) | Markings remain legible throughout the full product lifecycle—even after exposure to autoclaves or within the human body. |
| Tamper-Proof & Counterfeit-Resistant | Deep engravings and a cryptographically secured serial number system prevent removal, overmarking, or cloning. |
| UDI-Ready | Each marking includes all mandatory UDI data elements (DI + PI), forming the basis for integration with MES, ERP, or EUDAMED via scanners or vision systems. |
| Inline Verification-Ready | Open interfaces allow for seamless integration of cameras or handheld scanners—Senodis provides the “readable signature,” while customers choose how to connect it. |
Our technology creates micro-deep, high-contrast DataMatrix or human-readable codes directly on titanium, stainless steel, or CoCr surfaces—without compromising material integrity.
Real-World Implementation
| Process Step | Technical Integration | Direct Benefit |
|---|---|---|
| Implant Manufacturing (OEM) | Inline laser marking cell immediately after CNC finishing; serial numbers injected via CSV/MES call | Anti-counterfeit protection from day one, no labels required → 100% MDR-compliant UDI before passivation |
| Instrument Reprocessing (CSSD) | Mobile fiber laser workstation in sterile supply; operated via foot pedal and handheld scanner | In-hospital marking of legacy instruments without extra logistics – codes remain legible after 500 sterilization cycles |
| Goods Receipt (Hospital / Contract Processor) | Hand scanner or camera gate reads DataMatrix; unknown serials trigger quarantine | Prevents counterfeit deliveries, speeds up release and inventory booking |
Scope & Level
Pilot: 1 implant finishing cell (titanium hip stems), 1 laser & inspection station, 1 validation-grade autoclave, 1 CSSD partner clinic
Rollout: 3 manufacturing sites, 6 production lines,
4 CSSD service centers, 75,000 implants and 10,000 surgical instruments/yearSystem Interfaces: Laser/Scanner ↔ MES ↔ QMS, MES ↔ QMS ↔ ERP (SAP) → Bidirectional serial number verification
Stakeholders
QA/RA Manager:
MDR/FDA compliance, validation (IQ/OQ/PQ), audit readinessProduction Manager:
OEE ≥ 97% post-integration, seamless shopfloor integration of laser systemsIT / MES Admin:
Interface laser/scanner ↔ MES/ERP, data integrity, downtime ≤ 2 hours/yearSupply Chain Manager:
End-to-end implant & instrument tracking through surgery, loss rate < 0.5%Finance / Controlling:
ROI monitoring, target vs. actual savings tracking, board-level reportingKey Supplier (Laser & Scanner OEM):
Turn-key delivery, >95% equipment availability, operator training
Project Triggers
EU MDR Surveillance Audit in 9 Months
→ Permanently lasered UDI codes must be traceable and verifiable per deviceRecall Near Miss (Q1/2025)
→ Estimated risk exposure: €3.8M; urgent need for batch-level quarantine workflowsFDA Direct Marking Deadline for Class II Devices (Sept 2026)
→ Global rollout required; serial number format harmonization neededInvestment Program: “New Implant Platform 2025/26”
→ Laser marking can be integrated from day one instead of costly retrofitsBoard Directive: “Digital Traceability by 2026”
→ Budget and resources secured, with a firm project deadline
Key Metrics
Tray setup time per procedure
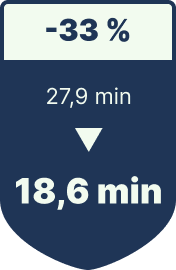
-33% reduction in setup time
Packaging defect rate
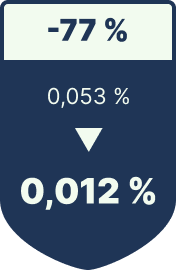
-77% reduction in failure costs
Readability after 500 sterilization cycles
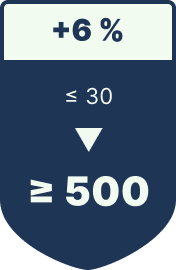
> 15x longer service life
Recall costs per incident

-99% capital risk
(Key Performance Indicator, delta, baseline, result)
Business Value & ROI
| Metric | Before | After | Annual Savings |
|---|---|---|---|
| OR Tray Setup Time | 27.9 min / surgery | 18.6 min | ≈ $281,000 saved through reduced OR idle time |
| Tape/Label Replacement | Retaping every ~30 cycles | Eliminated | ≈ €80,000 in materials and labor |
| Recall Exposure | Up to $600M worst-case | Batch-specific recall < $1M (projected) | >99% risk reduction |
| Packaging Defect Rework | 0.053% defect rate | 0.012% | ≈ €35,000 less rework |
Implementation Roadmap
| Phase | Duration | Key Stakeholders | Internal Effort |
|---|---|---|---|
| Feasibility Study & Material Testing | 4 weeks | QA/RA, Manufacturing Engineering | ≈ 8 person-days |
| Engineering, IQ/OQ, SOP Development | 12 weeks | Production, Equipment Engineering | ≈ 40 person-days |
| PQ & MDR Documentation | 8 weeks | QA/RA, Regulatory Affairs | ≈ 30 person-days |
| Roll-out & Training | Ongoing | CSSD, Logistics, IT | ≈ 0.1 FTE for support |